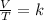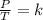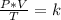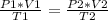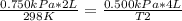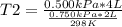Chemistry, 20.01.2021 01:50 KylaChanel4756
A sample of gas has a volume of 2.00 L and a pressure of 0.750 kPa when its
temperature is 25°C. If the volume is expanded to 4.00 L and the pressure reduced to
0.500 kPa, what must the temperature become?
379°C
397°C
379 K
397K

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
A sample of gas has a volume of 2.00 L and a pressure of 0.750 kPa when its
temperature is 25°C. If...
Questions

English, 26.08.2019 07:30

Mathematics, 26.08.2019 07:30







Mathematics, 26.08.2019 07:30

Mathematics, 26.08.2019 07:30

Biology, 26.08.2019 07:30

English, 26.08.2019 07:30

Mathematics, 26.08.2019 07:30

Biology, 26.08.2019 07:30

Geography, 26.08.2019 07:30



History, 26.08.2019 07:30

Mathematics, 26.08.2019 07:30