
Chemistry, 20.01.2021 04:40 alarimer3695
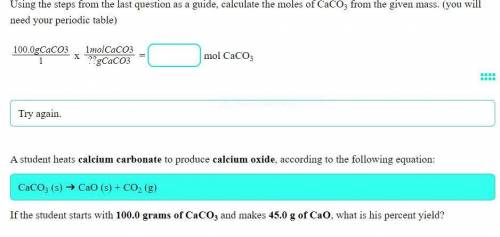
A student heats calcium carbonate to produce calcium oxide, according to the following equation:
CaCO3 (s) ➔ CaO (s) + CO2 (g)
If the student starts with 100.0 grams of CaCO3 and makes 45.0 g of CaO, what is his percent yield?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
A student heats calcium carbonate to produce calcium oxide, according to the following equation:
Ca...
Questions

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Health, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

History, 17.09.2020 21:01

History, 17.09.2020 21:01

Social Studies, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Physics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Spanish, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01

Spanish, 17.09.2020 21:01

Mathematics, 17.09.2020 21:01



