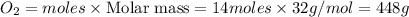Chemistry, 20.01.2021 17:20 Priskittles
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emissions such as carbon monoxide, CO(g), into carbon dioxide, CO2(g). The uncatalyzed reaction is represented by the balanced equation below.2CO(g) O2(g) 2CO2(g) +heat. determine the mass of O2(g) required to completely react with 784g moles of CO(g) during this reaction.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2


Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emission...
Questions

Mathematics, 08.07.2019 06:00


History, 08.07.2019 06:00




Biology, 08.07.2019 06:00



Mathematics, 08.07.2019 06:00

History, 08.07.2019 06:00

Mathematics, 08.07.2019 06:00



Biology, 08.07.2019 06:00


Mathematics, 08.07.2019 06:00



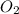 will be required to completely react with 784g moles of CO(g) during this reaction.
will be required to completely react with 784g moles of CO(g) during this reaction.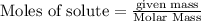
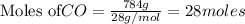
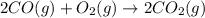
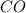 require = 1 mole of
require = 1 mole of 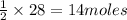 of
of