

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
13.How many grams of phosphorus (P4) are needed to completely consume 79.2 L of chlorine gas accordi...
Questions



Chemistry, 09.04.2021 01:40

Geography, 09.04.2021 01:40




Mathematics, 09.04.2021 01:40

World Languages, 09.04.2021 01:40


Mathematics, 09.04.2021 01:40

Mathematics, 09.04.2021 01:40



History, 09.04.2021 01:40



Spanish, 09.04.2021 01:40

SAT, 09.04.2021 01:40

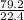 = 3.54mole
= 3.54mole 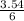 = 0.59mole of P₄
= 0.59mole of P₄ 

