
Chemistry, 21.01.2021 15:40 cxttiemsp021
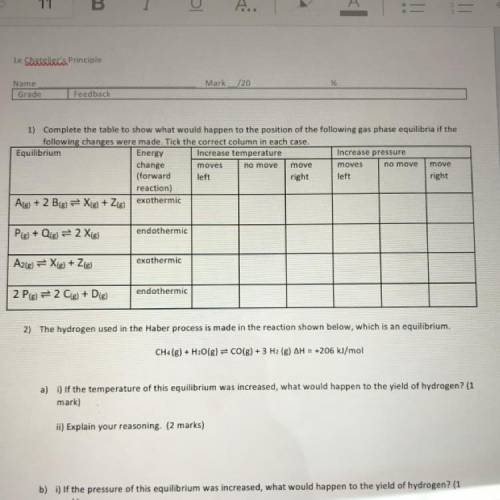
1) Complete the table to show what would happen to the position of the following gas phase equilibria if the
following changes were made. Tick the correct column in each case.
Equilibrium
Energy Increase temperature
Increase pressure
change
moves
moves
move
(forward left
right left
right
reaction)
Ale) + 2 B(g) = X(g) + Zig) exothermic
endothermic
Ple) + Qig) = 2 Xig)
exothermic
A2(g) = X(g) + 2(g)
endothermic
2 Pig) = 2 C(s) + D(g)

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
1) Complete the table to show what would happen to the position of the following gas phase equilibri...
Questions


Mathematics, 05.04.2021 20:10


Mathematics, 05.04.2021 20:10

Mathematics, 05.04.2021 20:10




Mathematics, 05.04.2021 20:10




Mathematics, 05.04.2021 20:10


Mathematics, 05.04.2021 20:10

Computers and Technology, 05.04.2021 20:10

Mathematics, 05.04.2021 20:10

Mathematics, 05.04.2021 20:10




