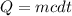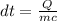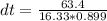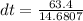If 63.4 J of heat are added to a sample
of aluminum with a mass of 16.33 g, what is
its tempe...


Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 2
You know the right answer?
Questions

Chemistry, 28.02.2021 07:50

Geography, 28.02.2021 07:50



Mathematics, 28.02.2021 07:50




Mathematics, 28.02.2021 07:50



Mathematics, 28.02.2021 07:50




Mathematics, 28.02.2021 07:50

Physics, 28.02.2021 07:50


History, 28.02.2021 07:50

Mathematics, 28.02.2021 07:50