
Chemistry, 21.01.2021 22:20 Jennifer312332
A 0.380 g sample of an unknown monoprotic acid is titrated with 0.300 M KOH to the equivalence point. It requires 20.1 mL of KOH to reach this point. Elemental analysis is also performed, showing that the acid contains 1.6% H, 22.2% N, and 76.2% O. Use this information to determine the molecular formula and the molar mass of the unknown acid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
A 0.380 g sample of an unknown monoprotic acid is titrated with 0.300 M KOH to the equivalence point...
Questions

Mathematics, 26.01.2021 18:00





Computers and Technology, 26.01.2021 18:00


Engineering, 26.01.2021 18:00

Mathematics, 26.01.2021 18:00

Arts, 26.01.2021 18:00


History, 26.01.2021 18:00

Chemistry, 26.01.2021 18:00


Biology, 26.01.2021 18:00


English, 26.01.2021 18:00

Physics, 26.01.2021 18:00


Mathematics, 26.01.2021 18:00

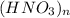 ( let )
( let ) 

