Chemistry, 21.01.2021 22:10 Gabysh4105
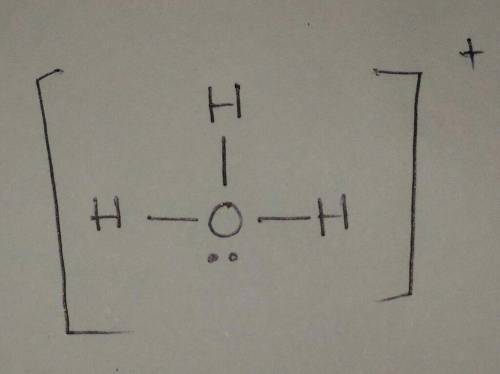
Draw a Lewis structure for H3O+ . Include all hydrogen atoms and show all unshared electrons and the formal charges, if any. Assume that bonding follows the octet rule.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Draw a Lewis structure for H3O+ . Include all hydrogen atoms and show all unshared electrons and the...
Questions

Mathematics, 07.01.2021 14:40


Mathematics, 07.01.2021 14:40

English, 07.01.2021 14:40

Mathematics, 07.01.2021 14:40

Mathematics, 07.01.2021 14:40

Mathematics, 07.01.2021 14:40

English, 07.01.2021 14:40

Mathematics, 07.01.2021 14:40

Mathematics, 07.01.2021 14:50



Mathematics, 07.01.2021 14:50

Biology, 07.01.2021 14:50

Mathematics, 07.01.2021 14:50

History, 07.01.2021 14:50

Physics, 07.01.2021 14:50

Mathematics, 07.01.2021 14:50

Biology, 07.01.2021 14:50