
Chemistry, 21.01.2021 22:20 stephensandrew4293
When the concentration of A in the reaction A B was changed from 1.20 M to 0.60 M, the half-life increased from 2.0 min to 4.0 min at 25°C. Calculate the order of the reaction and the rate constant.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 15:00
Solve this problem using the appropriate law. (remember that ) what is the pressure of 1.9 mols of nitrogen gas in a 9.45 l tank and at a temperature of 228 k?
Answers: 1

Chemistry, 23.06.2019 19:30
How has the scientific model of the atom changed over the centuries, and what new evidence led to the various changes in the model?
Answers: 1
You know the right answer?
When the concentration of A in the reaction A B was changed from 1.20 M to 0.60 M, the half-life in...
Questions


English, 18.01.2021 09:30

History, 18.01.2021 09:30

Mathematics, 18.01.2021 09:30

English, 18.01.2021 09:30

Advanced Placement (AP), 18.01.2021 09:30


Mathematics, 18.01.2021 09:30


Mathematics, 18.01.2021 09:30

Business, 18.01.2021 09:30

Biology, 18.01.2021 09:30



Mathematics, 18.01.2021 09:30



Chemistry, 18.01.2021 09:30

History, 18.01.2021 09:30

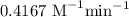
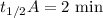
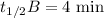
![{[A]_0}_A=1.2\ \text{M}](/tpl/images/1053/6371/ba19d.png)
![{[A]_0}_B=0.6\ \text{M}](/tpl/images/1053/6371/61cc0.png)
![t_{1/2}\propto \dfrac{1}{[A]_0^{n-1}}](/tpl/images/1053/6371/3ebb4.png)
![\dfrac{{t_{1/2}}_A}{{t_{1/2}}_B}=\left(\dfrac{{[A]_0}_B}{{[A]_0}_A}\right)^{n-1}\\\Rightarrow \dfrac{2}{4}=\left(\dfrac{0.6}{1.2}\right)^{n-1}\\\Rightarrow \dfrac{1}{2}=\left(\dfrac{1}{2}\right)^{n-1}](/tpl/images/1053/6371/86904.png)
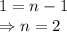
![t_{1/2}=\dfrac{1}{k[A]_0^{n-1}}\\\Rightarrow k=\dfrac{1}{t_{1/2}[A]_0^{n-1}}\\\Rightarrow k=\dfrac{1}{2\times 1.2^{2-1}}\\\Rightarrow k=0.4167\ \text{M}^{-1}\text{min}^{-1}](/tpl/images/1053/6371/f48ea.png)


