APPLY YOUR KNOWLEDGE
Graphite and diamond are different forms of the element carbon.
Graphite...

Chemistry, 22.01.2021 14:00 anacecilianr2325
APPLY YOUR KNOWLEDGE
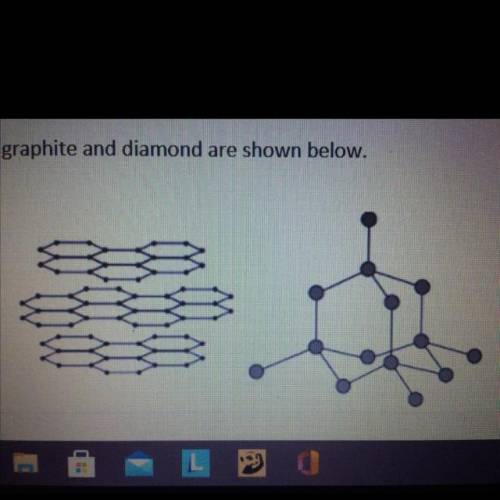
Graphite and diamond are different forms of the element carbon.
Graphite and diamond have different properties.
The structures of graphite and diamond are shown below.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
Questions


Computers and Technology, 11.09.2019 23:20



Computers and Technology, 11.09.2019 23:20



Computers and Technology, 11.09.2019 23:20

Mathematics, 11.09.2019 23:20




Mathematics, 11.09.2019 23:20





Chemistry, 11.09.2019 23:20


Social Studies, 11.09.2019 23:20



