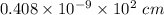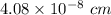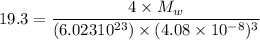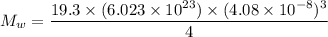Chemistry, 22.01.2021 19:00 sunshine0613
An element crystallizes in a face-centered cubic lattice. If the length of an edge of the unit cell is 0.408 nm, and the density of the element is 19.3 g/cm3 , what is the identity of the element?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
An element crystallizes in a face-centered cubic lattice. If the length of an edge of the unit cell...
Questions








Social Studies, 06.07.2019 09:00


Social Studies, 06.07.2019 09:00


Physics, 06.07.2019 09:00


Mathematics, 06.07.2019 09:00



Chemistry, 06.07.2019 09:00

Business, 06.07.2019 09:00

Advanced Placement (AP), 06.07.2019 09:00

Mathematics, 06.07.2019 09:00

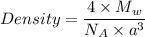
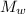 = molar mass of the compound
= molar mass of the compound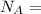 avogadro's constant
avogadro's constant 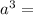 the volume of a unit cell
the volume of a unit cell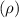 = 19.30 g/cm³
= 19.30 g/cm³