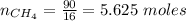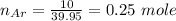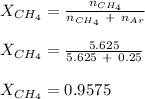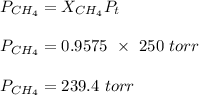Chemistry, 22.01.2021 20:00 ashleybashaam6821
A mixture of 90.0 grams of CH4 and 10.0 grams of argon has a pressure of 250 torr under conditions of constant temperature and volume. The partial pressure of CH4 in tore is?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
A mixture of 90.0 grams of CH4 and 10.0 grams of argon has a pressure of 250 torr under conditions o...
Questions

Mathematics, 24.02.2021 21:30

Mathematics, 24.02.2021 21:30

Mathematics, 24.02.2021 21:30

Mathematics, 24.02.2021 21:30


Mathematics, 24.02.2021 21:30


Mathematics, 24.02.2021 21:30


Mathematics, 24.02.2021 21:30

History, 24.02.2021 21:30

Mathematics, 24.02.2021 21:30

History, 24.02.2021 21:30

Physics, 24.02.2021 21:30


Chemistry, 24.02.2021 21:30

Chemistry, 24.02.2021 21:30

English, 24.02.2021 21:30

Mathematics, 24.02.2021 21:30

Health, 24.02.2021 21:30