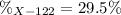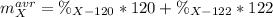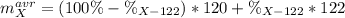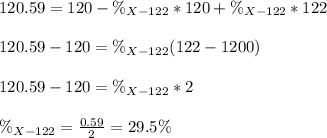Chemistry, 23.01.2021 07:40 Rockey3876
An unknown element is a mixture of isotopes ¹²⁰X and ¹²²X. The average atomic mass of X is 120.59 amu. What is the percent abundance of ¹²²X?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
An unknown element is a mixture of isotopes ¹²⁰X and ¹²²X. The average atomic mass of X is 120.59 am...
Questions

Mathematics, 29.01.2021 22:00


Mathematics, 29.01.2021 22:00

Mathematics, 29.01.2021 22:00


Biology, 29.01.2021 22:00


Mathematics, 29.01.2021 22:00


History, 29.01.2021 22:00


Biology, 29.01.2021 22:00



Mathematics, 29.01.2021 22:00



Spanish, 29.01.2021 22:00