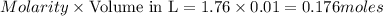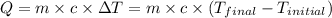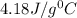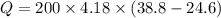Chemistry, 24.01.2021 14:00 shaheedbrown06
A reaction of 100mL of 1.35 M HCl and 100mL of 1.76 M NaOH is monitored and the following
temperatures were recorded: starting temperature = 24.6 C; and final temperature = 38.8 C.
Calculate the AH of this reaction.
A) -92142
J/Mole
B) -12439.2
J/Mole
C) 876
J/Mole

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
A reaction of 100mL of 1.35 M HCl and 100mL of 1.76 M NaOH is monitored and the following
temperatu...
Questions




Arts, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

Biology, 25.02.2021 01:10


Chemistry, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10


History, 25.02.2021 01:10

Mathematics, 25.02.2021 01:10

English, 25.02.2021 01:10

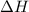 for the reaction is 87935 J
for the reaction is 87935 J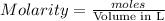
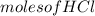 =
=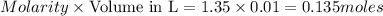
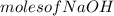 =
=