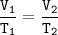Chemistry, 24.01.2021 14:00 gstinson98
A volume of 2.15 mL of Oxygen was collected in the lab at a temperature of 17 °C and 740.2 mmHg. After a day, the volume of the gas has become 2.21 mL and the barometer reading (for air pressure) had not changed. What is the new temperature in the lab (in °C)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
A volume of 2.15 mL of Oxygen was collected in the lab at a temperature of 17 °C and 740.2 mmHg. Aft...
Questions

Mathematics, 21.01.2020 09:31

Mathematics, 21.01.2020 09:31

Biology, 21.01.2020 09:31


English, 21.01.2020 09:31


Mathematics, 21.01.2020 09:31

History, 21.01.2020 09:31


Mathematics, 21.01.2020 09:31

Mathematics, 21.01.2020 09:31

Mathematics, 21.01.2020 09:31




Chemistry, 21.01.2020 09:31

Chemistry, 21.01.2020 09:31

English, 21.01.2020 09:31

Mathematics, 21.01.2020 09:31

Mathematics, 21.01.2020 09:31