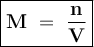A chemist prepares a solution of potassium iodide (KI) by measuring out 88. umol of potassium iodide into a 200. mL volumetric flask and filling the flask to
the mark with water.
Calculate the concentration in mol/L of the chemist's potassium iodide solution. Round your answer to 2 significant digits.
Please Help me!


Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
A chemist prepares a solution of potassium iodide (KI) by measuring out 88. umol of potassium iodide...
Questions

Health, 27.07.2021 05:30

Mathematics, 27.07.2021 05:30






Mathematics, 27.07.2021 05:30

Physics, 27.07.2021 05:30





Mathematics, 27.07.2021 05:30

Social Studies, 27.07.2021 05:30




English, 27.07.2021 05:30