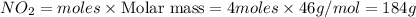Chemistry, 24.01.2021 23:20 jaksenpounders
Calculate the number of gams of nitrogen dioxide that are produced from 4 moles of nitric oxide
2NO(g) + O2(g) → 2NO2(g)
PLZ ANSWERRR FAST

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
Calculate the number of gams of nitrogen dioxide that are produced from 4 moles of nitric oxide
2NO...
Questions





Biology, 20.04.2020 23:25

Mathematics, 20.04.2020 23:25




Mathematics, 20.04.2020 23:25

Mathematics, 20.04.2020 23:25


History, 20.04.2020 23:25

Mathematics, 20.04.2020 23:25

Mathematics, 20.04.2020 23:25

Mathematics, 20.04.2020 23:25


Mathematics, 20.04.2020 23:25


Spanish, 20.04.2020 23:25

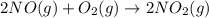
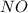 produce = 2 moles of
produce = 2 moles of 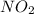
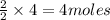 of
of