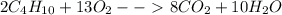Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
The balanced chemical equation below shows the combustion reaction of butane (C₄H₁₀). What is the mo...
Questions






Biology, 10.06.2021 21:10


English, 10.06.2021 21:10

Mathematics, 10.06.2021 21:10


Mathematics, 10.06.2021 21:10


English, 10.06.2021 21:10


Chemistry, 10.06.2021 21:10

Mathematics, 10.06.2021 21:10



Chemistry, 10.06.2021 21:10

Mathematics, 10.06.2021 21:10