
Chemistry, 25.01.2021 04:10 09daishagreen
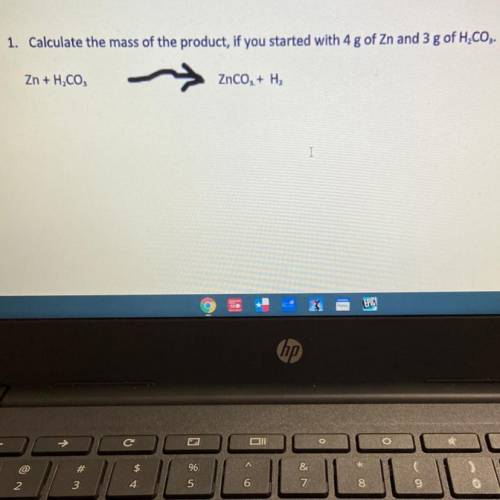
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H, CO,
ZnCO, + H2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
1. Calculate the mass of the product, if you started with 4 g of Zn and 3 g of H2CO3.
Zn + H, CO,
Questions


Mathematics, 28.08.2019 00:30


History, 28.08.2019 00:30


Mathematics, 28.08.2019 00:30

Chemistry, 28.08.2019 00:30

Physics, 28.08.2019 00:30

Chemistry, 28.08.2019 00:30

Advanced Placement (AP), 28.08.2019 00:30



Mathematics, 28.08.2019 00:30

French, 28.08.2019 00:30

Mathematics, 28.08.2019 00:30


Mathematics, 28.08.2019 00:30


Physics, 28.08.2019 00:30




