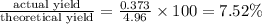Chemistry, 25.01.2021 18:00 MikeCrotch19251
2H2 (1) + O2(g) → 2H20 (g)
1. Find the limiting reactant if you start with 30.0 grams of hydrogen and 5.29 grams of oxygen.
2. The actual yield for H2O in the above reaction is 6.72 g, Determine the percent yield for the reaction
when 9.93 grams of hydrogen and excess oxygen react?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
2H2 (1) + O2(g) → 2H20 (g)
1. Find the limiting reactant if you start with 30.0 grams of hydrogen a...
Questions


Computers and Technology, 29.10.2019 21:31





Chemistry, 29.10.2019 21:31


Mathematics, 29.10.2019 21:31

Mathematics, 29.10.2019 21:31



History, 29.10.2019 21:31





Computers and Technology, 29.10.2019 21:31


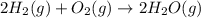
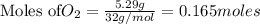
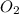 require = 2 moles of
require = 2 moles of 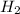
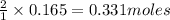 of
of 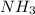
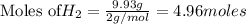
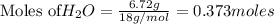
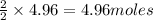 of
of