
Chemistry, 25.01.2021 18:00 kromaug7986
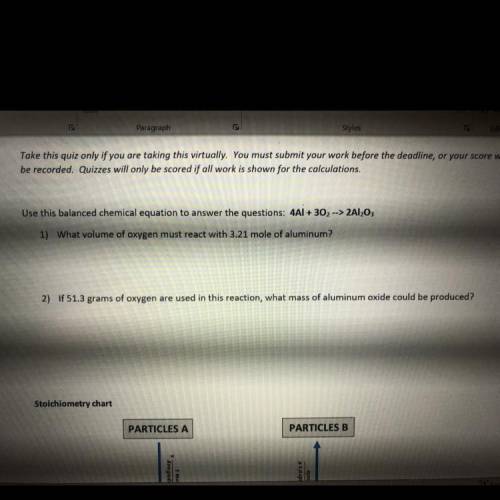
2) If 51.3 grams of oxygen are used in this reaction, what mass of aluminum oxide could be produced?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
2) If 51.3 grams of oxygen are used in this reaction, what mass of aluminum oxide could be produced?...
Questions

Chemistry, 18.03.2021 03:30

Health, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

Chemistry, 18.03.2021 03:30


Computers and Technology, 18.03.2021 03:30

Physics, 18.03.2021 03:30





Arts, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30


English, 18.03.2021 03:30


Geography, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30



