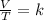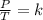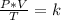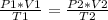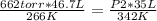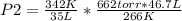Chemistry, 25.01.2021 18:10 Isaiahtate053
3) 46.7 L of a gas is initially at a pressure of 662 torr and a temperature of 266 K. If the volume decreases to 35.0 L and the temperature increases to 342 K, what is the new pressure?

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 16:00
Express your answer using two significant figures. 1.7 km^2
Answers: 1

Chemistry, 23.06.2019 17:30
Describe the type of force which exists between particles in an ideal gas. explain why this type of force exists between the particles.
Answers: 3
You know the right answer?
3) 46.7 L of a gas is initially at a pressure of 662 torr and a temperature of 266 K. If the volume...
Questions

English, 25.01.2021 23:10


Mathematics, 25.01.2021 23:10



Mathematics, 25.01.2021 23:10





History, 25.01.2021 23:10






Mathematics, 25.01.2021 23:10

Mathematics, 25.01.2021 23:10

History, 25.01.2021 23:10