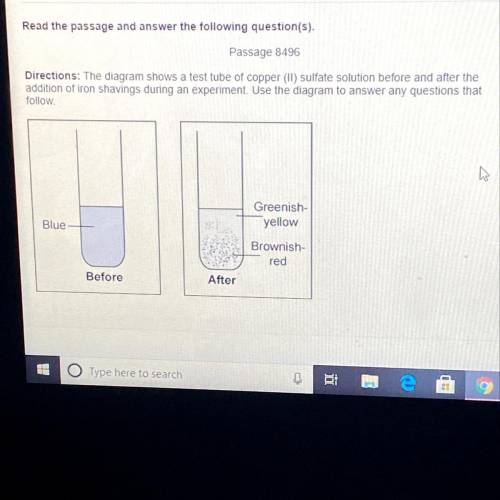
The experiment shows a-
A. chemical reaction, because the solution changed the color
B...

Chemistry, 25.01.2021 21:00 abdirisak33
The experiment shows a-
A. chemical reaction, because the solution changed the color
B. physical reaction, because the solution changed the color
C. physical reaction, because it involved a compound
D. chemical reaction, because it involved a mixture

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2
You know the right answer?
Questions

Mathematics, 23.04.2020 23:25

Chemistry, 23.04.2020 23:25



Biology, 23.04.2020 23:25



Mathematics, 23.04.2020 23:25





Mathematics, 23.04.2020 23:25






History, 23.04.2020 23:25



