Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
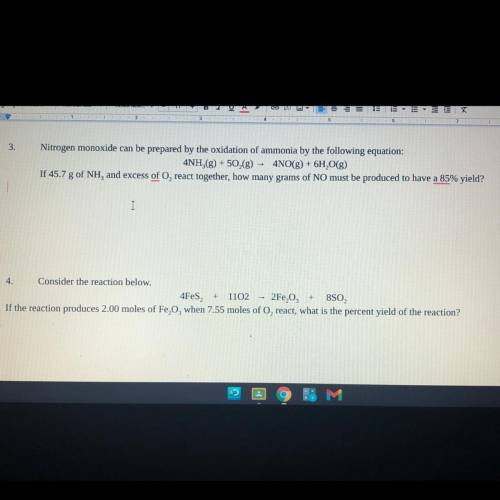
Consider the reaction below.
4Fes2 + 11O2 —> 2Fe2O3 + 8SO2
If the reaction produces 2.00 m...
If the reaction produces 2.00 m...
Questions







World Languages, 21.07.2019 21:30

Chemistry, 21.07.2019 21:30


Biology, 21.07.2019 21:30


Biology, 21.07.2019 21:30


Biology, 21.07.2019 21:30


Biology, 21.07.2019 21:30


History, 21.07.2019 21:30


History, 21.07.2019 21:30