
Chemistry, 26.01.2021 05:40 kenneth0125
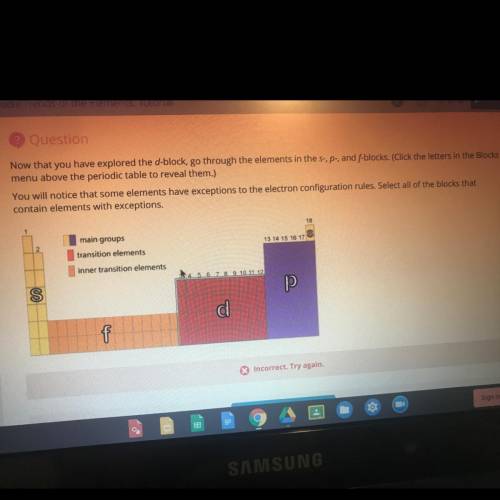
Now that you have explored the d-block, go through the elements in the s-, p-, and f-blocks. You will notice that some elements have exceptions to the electron configuration rules. Select all of the blocks that contain elements with exceptions.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Now that you have explored the d-block, go through the elements in the s-, p-, and f-blocks.
You wi...
Questions

History, 14.12.2019 20:31

Mathematics, 14.12.2019 20:31

History, 14.12.2019 20:31


Mathematics, 14.12.2019 20:31

Geography, 14.12.2019 20:31

History, 14.12.2019 20:31






Biology, 14.12.2019 20:31


Mathematics, 14.12.2019 20:31

Computers and Technology, 14.12.2019 20:31




Social Studies, 14.12.2019 20:31



