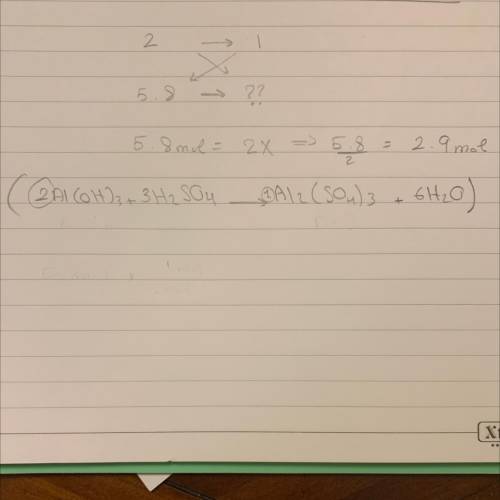Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2


Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Based on the equation below, how many moles of aluminum sulfate (Al2(SO4)3) will be produced from th...
Questions

Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00



Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00



Mathematics, 30.11.2020 20:00


Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00



Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00

Mathematics, 30.11.2020 20:00



English, 30.11.2020 20:00