
(05.06) % Yield Lab Report
1. Write the balanced chemical equation for the reaction you’re performing
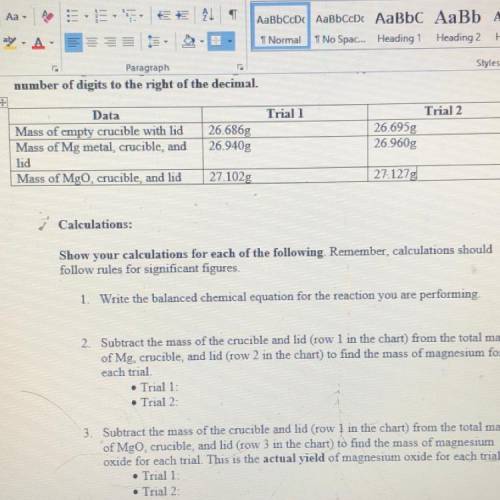
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and kid (row 2 in the chart) to find the mass of magnesium for each trial
Trial 1:
Trial 2:
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual YIELD of magnesium oxide for each trial
Trial 1:
Trial 2:
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial
Trial 1:
Trial 2:
6. Determine the average percent yield of MgO for 2 Trials
CONCLUSION
Write a conclusion statement that addresses the following questions
• Explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
• What sources of error may have contributed to the percent yield not being 100%?
(Think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.)
• How do you think the investigation can be explored further?
POST LAB REFLECTION QUESTIONS
1. When conducting this experiment, some procedures call for heating the substance several times and recording the mass after each heating, continuing until the mass values are constant. Explain the purpose of this process and how it might reduce errors.
2. Your company currently uses a process with a similar cost of materials that has an average percent yield of 91%. If the average percent yield of this process is higher than that, this could save the company money. What is your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 21:20
Juju.01) 5 geologic events a group of students designed an experiment in an ice rink to represent the solar system. the steps of the experiment are listed below. 5: geologic events 1. choose a student to represent the sun. the planets are represented by two tennis balls. 2. ask the student to hold the tennis balls in each palm and spin on the ice with arms stretched out. 3. ask the student to draw in the arms after about 10 spins. 4. observe the student's arms rotate faster when they are closer to the body. 05 geologic events enors) the experiment most likely demonstrates that (2 points) 07 discussion-based sessment/module planets exert gravitational force on the sun the sun exerts gravitational force on the planets 3.07 discussion-based ssessment speed of a planet depends on its distance from the sun new version available! (3.0.119) get it now submit 18.07: module exam description
Answers: 3
You know the right answer?
(05.06) % Yield Lab Report
1. Write the balanced chemical equation for the reaction you’re performi...
Questions

Arts, 20.04.2021 07:20





Physics, 20.04.2021 07:20




Social Studies, 20.04.2021 07:20




Mathematics, 20.04.2021 07:20

Mathematics, 20.04.2021 07:20

Mathematics, 20.04.2021 07:20

Biology, 20.04.2021 07:20


Mathematics, 20.04.2021 07:20




