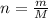In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 112. liters per second of dinitroge are consumed when the reaction is run at 17.2°C and 0.88atm.
Required:
Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with...
Questions


Geography, 28.06.2019 11:10

Mathematics, 28.06.2019 11:10





History, 28.06.2019 11:10

Mathematics, 28.06.2019 11:10



Mathematics, 28.06.2019 11:10

Mathematics, 28.06.2019 11:10

Social Studies, 28.06.2019 11:10

Mathematics, 28.06.2019 11:20


History, 28.06.2019 11:20



Mathematics, 28.06.2019 11:20

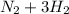 →
→ 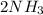
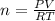
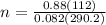
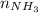 = 2(4.14)
= 2(4.14)