
Chemistry, 27.01.2021 21:20 andrewcassity1
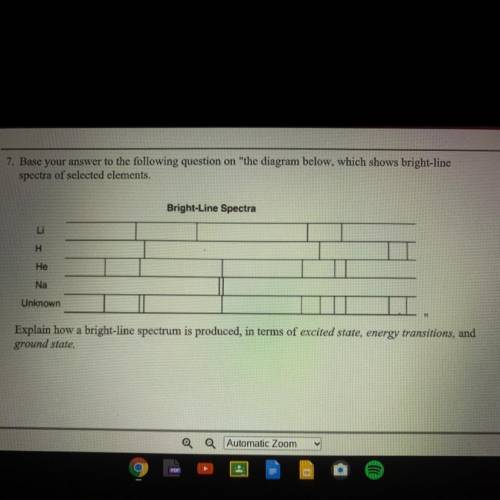
Explain how a bright-line spectrum is produced, in terms of excited state, energy transitions, and ground state.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1


Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1
You know the right answer?
Explain how a bright-line spectrum is produced, in terms of excited state, energy transitions, and...
Questions


English, 17.10.2019 12:00

Mathematics, 17.10.2019 12:00

History, 17.10.2019 12:00



Mathematics, 17.10.2019 12:00

Mathematics, 17.10.2019 12:00




Mathematics, 17.10.2019 12:00




Arts, 17.10.2019 12:00

History, 17.10.2019 12:00





