
I have a very lucrative offer for you if you answer the questions:
The volume of a 67.2-gram sample of platinum with a density of 21.5 g/cm3 is
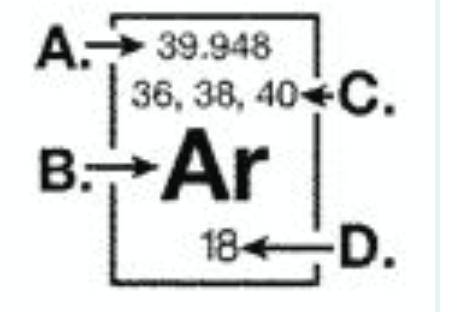
The diagram below represents information that can be found on the periodic table for the element argon. The mass number for an argon isotope is represented at: (graph is attached)
The sum of the atomic mass values of the atoms in a chemical formula is known as the
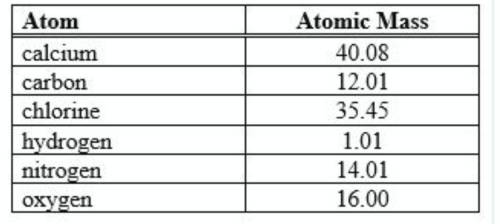
Using the table for reference, find the formula mass for nitric acid, HNO3. (table is attached)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1


Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
I have a very lucrative offer for you if you answer the questions:
The volume of a 67.2-gram sample...
Questions



Mathematics, 31.08.2019 16:50



Mathematics, 31.08.2019 16:50

Mathematics, 31.08.2019 16:50



Geography, 31.08.2019 16:50


History, 31.08.2019 16:50


English, 31.08.2019 16:50

Mathematics, 31.08.2019 16:50

Mathematics, 31.08.2019 16:50

Mathematics, 31.08.2019 16:50





