
Chemistry, 28.01.2021 03:50 kristygodwin
Pls Pls Pls help me asap, I'm so confused
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
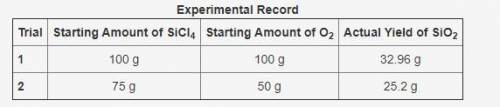
The table shows an experimental record for the above reaction. (table in pic)
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 13:00
Using the periodic table complete the table to describe each atom type in your answers
Answers: 1

Chemistry, 23.06.2019 14:00
What can happen to an atoms electrons when an electric current is passed through the atom?
Answers: 1
You know the right answer?
Pls Pls Pls help me asap, I'm so confused
In the following reaction, oxygen is the excess reactant....
Questions




Spanish, 12.10.2019 01:10


English, 12.10.2019 01:10




Mathematics, 12.10.2019 01:10









History, 12.10.2019 01:10

Medicine, 12.10.2019 01:10



