Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CC...

Chemistry, 28.01.2021 06:40 zoelynn8386
Consider the equations below.
CH4 (g)->C(s)+2H2(g) H1 = 74.6 kJ
C(s) + 2CI2(g)->CCI4(g) H2 = -95.7 kJ
2H2(g) + 2CI2(g)-> 4HCI(g) H3 = -184.6 kJ
CH4(g) + 4CI2(g) -> CCI4(g) + 4HCI(g) H4 = -205.7 kJ
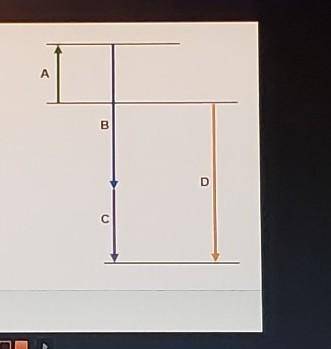
Complete the following based on the diagram.
Arrow A: 74.6 kJ
-95.7 kJ
-184.6 kJ
Arrow B: endothermic
exothermic
Arrow C: - bas a magnitude that is greater than that of B
- has a magnitude that is less than that of B
- has negative enthalpy
Arrow D: - represents an intermediate reaction
- has a magnitude that is always higher than any intermediate reaction
- represents the overall enthalpy of reaction

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Questions





History, 31.03.2020 06:48

English, 31.03.2020 06:48


Social Studies, 31.03.2020 06:48

Geography, 31.03.2020 06:48


History, 31.03.2020 06:49

Mathematics, 31.03.2020 06:49


English, 31.03.2020 06:49

Mathematics, 31.03.2020 06:49


Mathematics, 31.03.2020 06:49

Mathematics, 31.03.2020 06:49

Spanish, 31.03.2020 06:49

Chemistry, 31.03.2020 06:49



