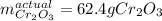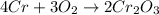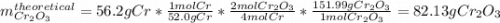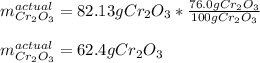Chemistry, 28.01.2021 08:00 madiballet125
Please help me understand how to...
Calculate the mass of Cr2O3 that can be produced if the reaction of 56.2 g of chromium and sufficient oxygen has a 76.0 % yield.
...Thank you!

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2


Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
Please help me understand how to...
Calculate the mass of Cr2O3 that can be produced if the reactio...
Questions

Chemistry, 25.01.2022 01:30

Chemistry, 25.01.2022 01:30

Physics, 25.01.2022 01:30


Mathematics, 25.01.2022 01:30

Computers and Technology, 25.01.2022 01:30

Geography, 25.01.2022 01:30

English, 25.01.2022 01:30

Mathematics, 25.01.2022 01:30




Mathematics, 25.01.2022 01:30

Chemistry, 25.01.2022 01:30

History, 25.01.2022 01:30


Mathematics, 25.01.2022 01:30


History, 25.01.2022 01:30

Biology, 25.01.2022 01:30