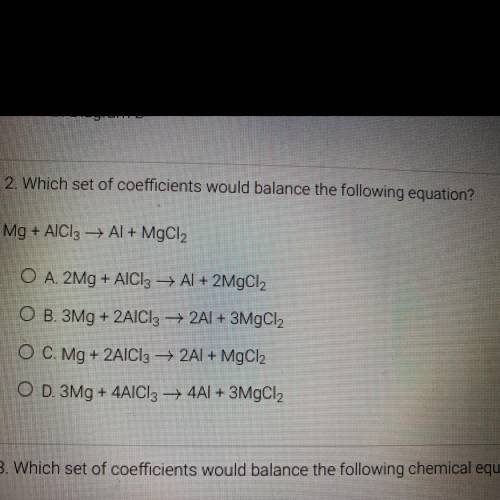Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
2. Which set of coefficients would balance the following equation?
Mg + AlCl3 + Al + MgCl2
O...
O...
Questions





Mathematics, 28.08.2020 22:01



Computers and Technology, 28.08.2020 22:01


Mathematics, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01


Mathematics, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01

History, 28.08.2020 22:01



English, 28.08.2020 22:01