Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

You know the right answer?
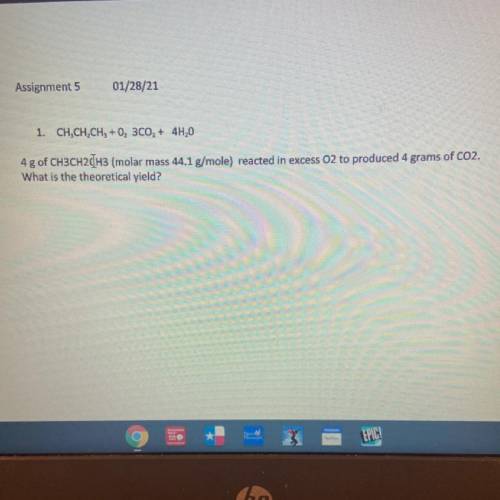
1. CH, CH, CH3 + 0, 300, + 4H,0
4g of CH3CH2CH3 (molar mass 44.1 g/mole) reacted in excess O2 to pr...
Questions


Mathematics, 17.11.2020 17:40

Biology, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40

History, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40

History, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40


Mathematics, 17.11.2020 17:40


History, 17.11.2020 17:40

English, 17.11.2020 17:40



Mathematics, 17.11.2020 17:40

Spanish, 17.11.2020 17:40


Mathematics, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40