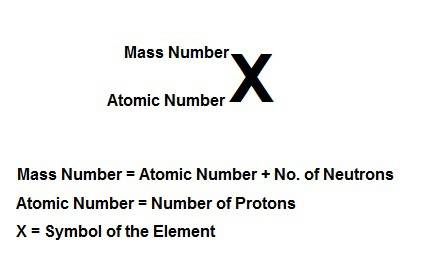
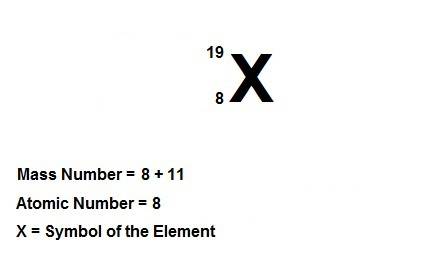Write the symbolic notation of an isotope of an element having 8 protons, 8 electrons, and 11 neutrons. click on the “templates” button template button and make use of the "stacked super/subscript" button button for entering stackes super/subscripts for entering the mass number and atomic number of the isotope. express your answer as an isotope.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Write the symbolic notation of an isotope of an element having 8 protons, 8 electrons, and 11 neutro...
Questions

Mathematics, 28.02.2021 04:20

Mathematics, 28.02.2021 04:20


Computers and Technology, 28.02.2021 04:20

Mathematics, 28.02.2021 04:20

Mathematics, 28.02.2021 04:20





Health, 28.02.2021 04:20

Mathematics, 28.02.2021 04:20


English, 28.02.2021 04:20


Mathematics, 28.02.2021 04:20


Geography, 28.02.2021 04:20

Mathematics, 28.02.2021 04:20

History, 28.02.2021 04:20