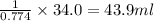Chemistry, 29.01.2021 16:30 elisakgarcia2007
The solubility in water of ionic compound X is measured and found to be 0.776g/mL at 15.°C. Calculate the volume of a saturated solution of X in water that would contain 34.0g of X at this temperature. Be sure your answer has the correct unit symbol and 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1

Chemistry, 23.06.2019 07:00
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2

Chemistry, 23.06.2019 09:00
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
You know the right answer?
The solubility in water of ionic compound X is measured and found to be 0.776g/mL at 15.°C. Calculat...
Questions

Mathematics, 12.07.2019 09:30


Mathematics, 12.07.2019 09:30

History, 12.07.2019 09:30

Biology, 12.07.2019 09:30




Mathematics, 12.07.2019 09:30


Social Studies, 12.07.2019 09:30

Social Studies, 12.07.2019 09:30




Computers and Technology, 12.07.2019 09:30

Biology, 12.07.2019 09:30


History, 12.07.2019 09:30

Mathematics, 12.07.2019 09:30

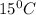 = 0.776 g/ml
= 0.776 g/ml