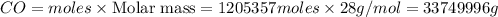The multistep smelting of ferric oxide to form elemental iron occurs at high temperatures in a blast furnace. In the first step, ferric oxide reacts with carbon monoxide to form Fe₃O.₄ This substance reacts with more carbon monoxide to formiron(II) oxide, which reacts with still more carbon monoxide to form molten iron. Carbon dioxide is also produced in each step.
(a) Write an overall balanced equation for the iron-smelting process.
(b) How many grams of carbon monoxide are required to form 45.0 metric tons of iron from ferric oxide?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2


Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
The multistep smelting of ferric oxide to form elemental iron occurs at high temperatures in a blast...
Questions


Spanish, 03.11.2020 14:00

English, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00


Health, 03.11.2020 14:00




Business, 03.11.2020 14:00



Mathematics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00


Mathematics, 03.11.2020 14:00

Mathematics, 03.11.2020 14:00



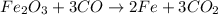
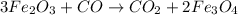
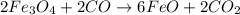
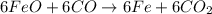
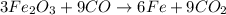
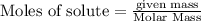
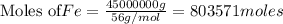
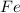 are produced from= 3 moles of
are produced from= 3 moles of 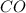
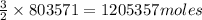 of
of