
Chemistry, 29.01.2021 19:40 adamgala3885
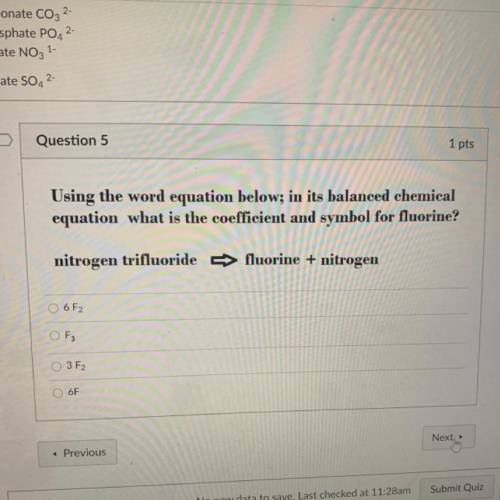
Using the word equation below; in its balanced chemical
equation what is the coefficient and symbol for fluorine?
nitrogen trifluoride -> fluorine + nitrogen
6F2
O F3
3 F2
6F

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2
You know the right answer?
Using the word equation below; in its balanced chemical
equation what is the coefficient and symbol...
Questions




Mathematics, 19.04.2020 23:55

Mathematics, 19.04.2020 23:55


Mathematics, 19.04.2020 23:55

Law, 19.04.2020 23:55

Mathematics, 19.04.2020 23:55

Biology, 19.04.2020 23:55

Mathematics, 19.04.2020 23:55

Mathematics, 19.04.2020 23:56





Mathematics, 19.04.2020 23:56

History, 19.04.2020 23:56

Mathematics, 19.04.2020 23:56

Mathematics, 19.04.2020 23:56



