
Chemistry, 29.01.2021 21:40 IkweWolf1824
Given 12.94 L of oxygen gas at STP, how many grams of iron (iii) oxide would be produced with excess iron? Round to two decimal places.
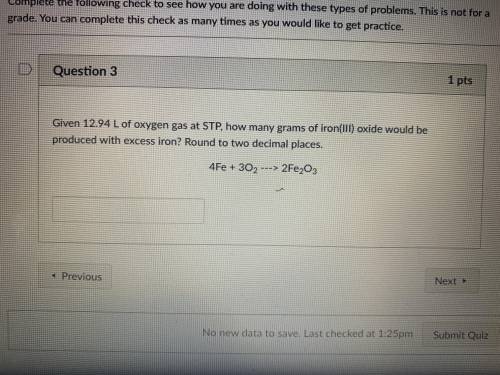

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 07:00
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2
You know the right answer?
Given 12.94 L of oxygen gas at STP, how many grams of iron (iii) oxide would be produced with excess...
Questions


Mathematics, 23.04.2021 07:30

Mathematics, 23.04.2021 07:30

Mathematics, 23.04.2021 07:30



Physics, 23.04.2021 07:30



Mathematics, 23.04.2021 07:30

Mathematics, 23.04.2021 07:30


Mathematics, 23.04.2021 07:30

Mathematics, 23.04.2021 07:30

Mathematics, 23.04.2021 07:30


Mathematics, 23.04.2021 07:30


English, 23.04.2021 07:30

Mathematics, 23.04.2021 07:30



