
Chemistry, 30.01.2021 05:40 dominguezjose625
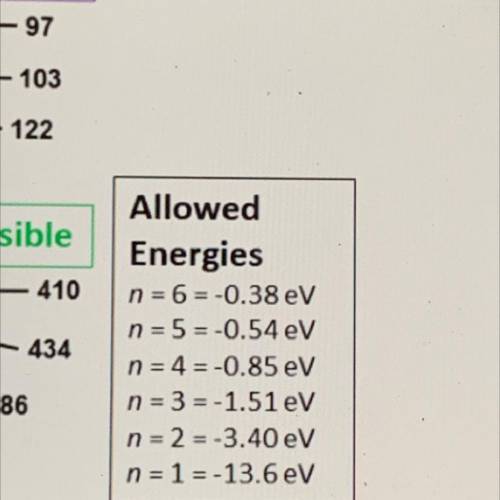
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-6 in orange and black. The black numbers
are the wavelengths in nanometers of the associated electromagnetic radiation.
Which wavelength is associated with a photon energy of 1.13 electron volts?
Bohr Model of the Hydrogen Atom
ultraviolet
infrared
-97
103
1,094
122
1,282
1,875
visible
410
434
Allowed
Energies
n = 6-0.38 eV
n = 5=-0.54 eV
n = 4 = -0.85 eV
n = 3 = -1.51 eV
n = 2 = -3.40 eV
n = 1 = -13.6 eV
486
656
In orbits
n orbits

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-...
Questions

Health, 04.02.2020 10:58



Geography, 04.02.2020 10:58



History, 04.02.2020 10:58

Mathematics, 04.02.2020 10:58

Business, 04.02.2020 10:58

English, 04.02.2020 10:58

Mathematics, 04.02.2020 10:58


English, 04.02.2020 10:58


Mathematics, 04.02.2020 10:58


History, 04.02.2020 10:58


History, 04.02.2020 10:58



