
Chemistry, 30.01.2021 05:40 hdjsjfjruejchhehd
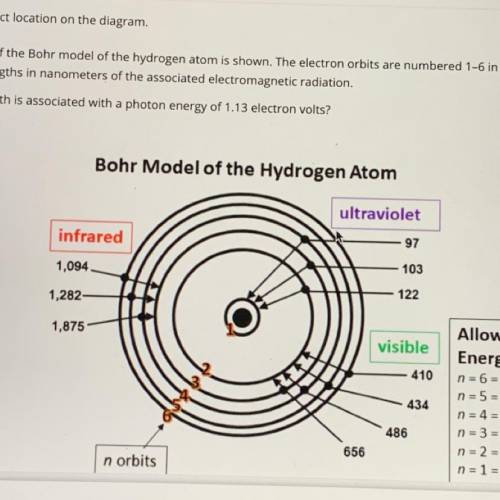
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-6 in orange and black. The black numbers are the wavelengths in nanometers of the associated electromagnetic radiation. Which wavelength is associated with a photon energy of 1.13 electron volts?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

Chemistry, 23.06.2019 08:40
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
You know the right answer?
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-...
Questions


Mathematics, 27.10.2019 18:43


Mathematics, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43


Mathematics, 27.10.2019 18:43

English, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43


Mathematics, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43


English, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43


Mathematics, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43

Mathematics, 27.10.2019 18:43

Biology, 27.10.2019 18:43



