
Chemistry, 30.01.2021 06:10 iamabouttofail
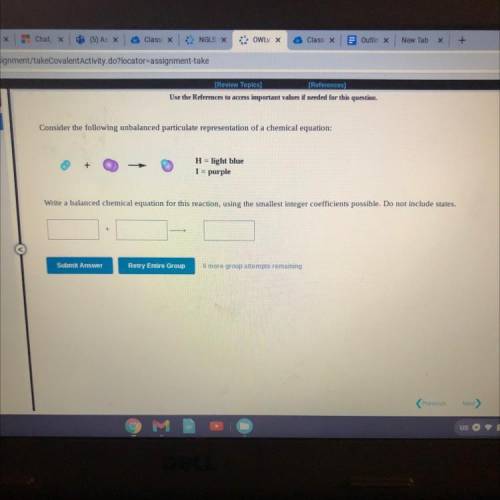
Consider the following unbalanced particulate representation of a chemical equation:
H = light blue
I = purple
Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1


You know the right answer?
Consider the following unbalanced particulate representation of a chemical equation:
H = light blue...
Questions

Mathematics, 08.01.2021 16:50


Chemistry, 08.01.2021 16:50

History, 08.01.2021 16:50

Social Studies, 08.01.2021 16:50


Mathematics, 08.01.2021 16:50





Geography, 08.01.2021 16:50







English, 08.01.2021 16:50

Mathematics, 08.01.2021 16:50



