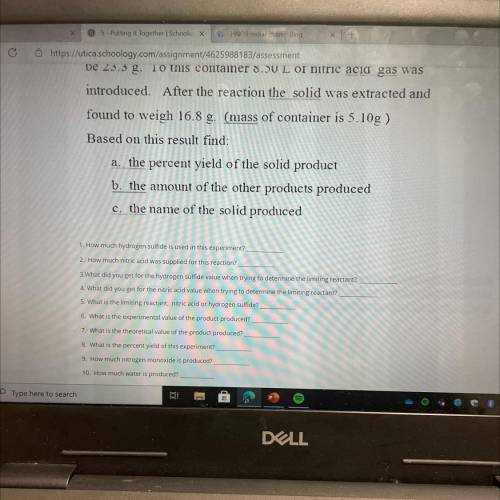
Elemental sulfur is one of the products of the gas-phase
reaction of nitric acid and hydrogen...

Chemistry, 30.01.2021 07:30 jnsebastian2002
Elemental sulfur is one of the products of the gas-phase
reaction of nitric acid and hydrogen sulfide. The other
products are nitrogen monoxide(g) and water(g). A
container and hydrogen sulfide (s) are massed and found to
be 23.3 g. To this container 8.50 L of nitric acid gas was
introduced. After the reaction the solid was extracted and
found to weigh 16.8 g. (mass of container is 5.10g )
Based on this result find:
a. the percent yield of the solid product
b. the amount of the other products produced
c. the name of the solid produced
1 How much hydrogen sulfide is used in this experiment?

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1


You know the right answer?
Questions



Mathematics, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01





History, 16.10.2020 08:01

History, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01



Physics, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01



