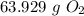Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
CH4 + 202 → CO2 + 2H2O
How many grams of O2 needed to produce 36 grams of H2O?...
How many grams of O2 needed to produce 36 grams of H2O?...
Questions

Mathematics, 11.03.2021 15:20

Mathematics, 11.03.2021 15:20

Spanish, 11.03.2021 15:20

Mathematics, 11.03.2021 15:20

Physics, 11.03.2021 15:20

Mathematics, 11.03.2021 15:30


Mathematics, 11.03.2021 15:30

Mathematics, 11.03.2021 15:30



Mathematics, 11.03.2021 15:30



Mathematics, 11.03.2021 15:30

Mathematics, 11.03.2021 15:30




Social Studies, 11.03.2021 15:30

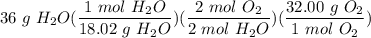 Divide/Multiply [Cancel Units]:
Divide/Multiply [Cancel Units]: