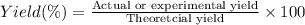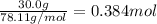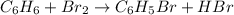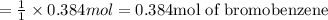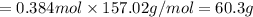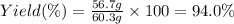Chemistry, 31.01.2021 04:40 ligittiger12806
1.What is the theoretical yield of C6H5Br in this reaction when 30.0 g of C6H6 reacts with excess Br2? If the actual yield of C6H5Br was 56.7 g, what is the percent yield?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
1.What is the theoretical
yield of C6H5Br in this reaction when 30.0 g of C6H6
reacts with excess B...
Questions

Biology, 04.12.2020 02:40





Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40



Mathematics, 04.12.2020 02:40


Social Studies, 04.12.2020 02:40


History, 04.12.2020 02:40




Mathematics, 04.12.2020 02:40

History, 04.12.2020 02:40