Calculate the mass percent of Calcium Chloride in
5.00g of CaCl2 in 95.0 g of water...


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Questions

Business, 19.12.2020 23:30



History, 19.12.2020 23:30


Mathematics, 19.12.2020 23:30

History, 19.12.2020 23:30

Mathematics, 19.12.2020 23:30

Mathematics, 19.12.2020 23:30

Mathematics, 19.12.2020 23:30


Mathematics, 19.12.2020 23:30



Biology, 19.12.2020 23:30

Mathematics, 19.12.2020 23:30


Social Studies, 19.12.2020 23:30

Mathematics, 19.12.2020 23:40

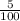 x 100 = 5%
x 100 = 5%

