
Chemistry, 31.01.2021 15:40 ultimatesaiyan
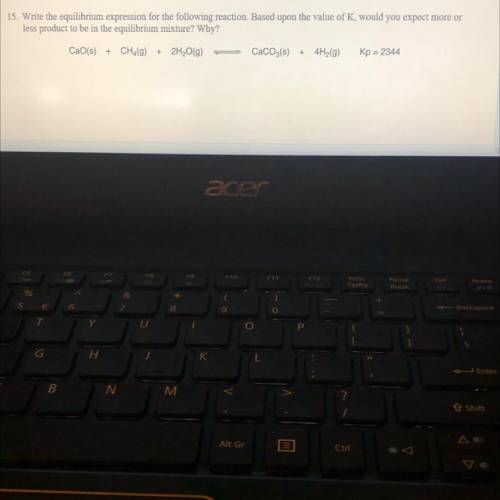
Write the equilibrium expression for the following reaction Based upon the value of K, would you expect more on
less product to be in the equilibrium mixture? Why?
COs) + CH (g)
+ 2H2O(g)
Caco_(s) +
Kp = 2344

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
Write the equilibrium expression for the following reaction Based upon the value of K, would you exp...
Questions







Medicine, 30.03.2021 19:10




Mathematics, 30.03.2021 19:10


Social Studies, 30.03.2021 19:10

Mathematics, 30.03.2021 19:10


Mathematics, 30.03.2021 19:10

Mathematics, 30.03.2021 19:10


Advanced Placement (AP), 30.03.2021 19:10

History, 30.03.2021 19:10



