
Chemistry, 31.01.2021 23:10 willcohen42
1. How many moles of CO2 can be produced from a reaction of 10.0 moles CzHg? 2C₂H6 + 702 4 CO2 + 6H₂O

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
1. How many moles of CO2 can be produced from a reaction of 10.0 moles CzHg?
2C₂H6 + 702 4 CO2 + 6H...
Questions

Health, 10.11.2020 05:20

Mathematics, 10.11.2020 05:20



Spanish, 10.11.2020 05:20

Social Studies, 10.11.2020 05:20

Chemistry, 10.11.2020 05:20

English, 10.11.2020 05:20


Mathematics, 10.11.2020 05:20

History, 10.11.2020 05:20


Mathematics, 10.11.2020 05:20


Mathematics, 10.11.2020 05:20





History, 10.11.2020 05:20

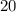 moles of CO2 can be produced from a reaction of 10.0 moles C2H6
moles of CO2 can be produced from a reaction of 10.0 moles C2H6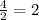 moles of Carbon dioxide (CO2)
moles of Carbon dioxide (CO2)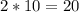 moles of Carbon dioxide (CO2)
moles of Carbon dioxide (CO2)

