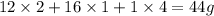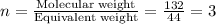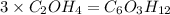Chemistry, 01.02.2021 14:00 ciaotaylor
A compound is determined to have the empirical formula C2OH4. If the molar mass of the compound is 132 g/mol, determine the molecular formula of the compound.


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
A compound is determined to have the empirical formula C2OH4. If the molar mass of the compound is 1...
Questions

Mathematics, 26.08.2020 04:01

Mathematics, 26.08.2020 04:01


Chemistry, 26.08.2020 04:01


Mathematics, 26.08.2020 04:01



Physics, 26.08.2020 04:01


Mathematics, 26.08.2020 04:01

Advanced Placement (AP), 26.08.2020 04:01


Chemistry, 26.08.2020 04:01


Chemistry, 26.08.2020 04:01

Geography, 26.08.2020 04:01


Social Studies, 26.08.2020 04:01

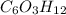
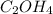 is
is